Pharmaceutical Innovation Management
Life Sciences Sector
Pharmaceutical companies face mounting challenges including high R&D costs, slow AI adoption, complex regulatory environments, and global market entry hurdles. KanBo provides a centralized, structured platform that empowers life sciences organizations to streamline innovation, ensure compliance, and drive operational efficiency across R&D, regulatory, and commercial functions.
Company’s Overview
Industry:
Pharmaceutical & Biotechnology
Key Focus Areas:
AI integration, R&D efficiency, regulatory compliance, market expansion
Size:
Global pharmaceutical leader with research hubs in the U.S., Europe, and Asia
Challenges:
High development costs, slow AI implementation, regulatory complexity, market access delays
Stakeholder Perspective
Chief Innovation Officer (CIO)
Our biggest hurdle is the cost and complexity of drug development. AI and digital transformation promise efficiency, yet implementation remains slow. Regulatory uncertainty also makes pricing and market entry timelines unpredictable. We need a centralized platform that streamlines our processes, integrates AI workflows, and ensures compliance.
Get started today with KanBo!
You can see KanBo in action by accessing our Sandbox demonstration environment.
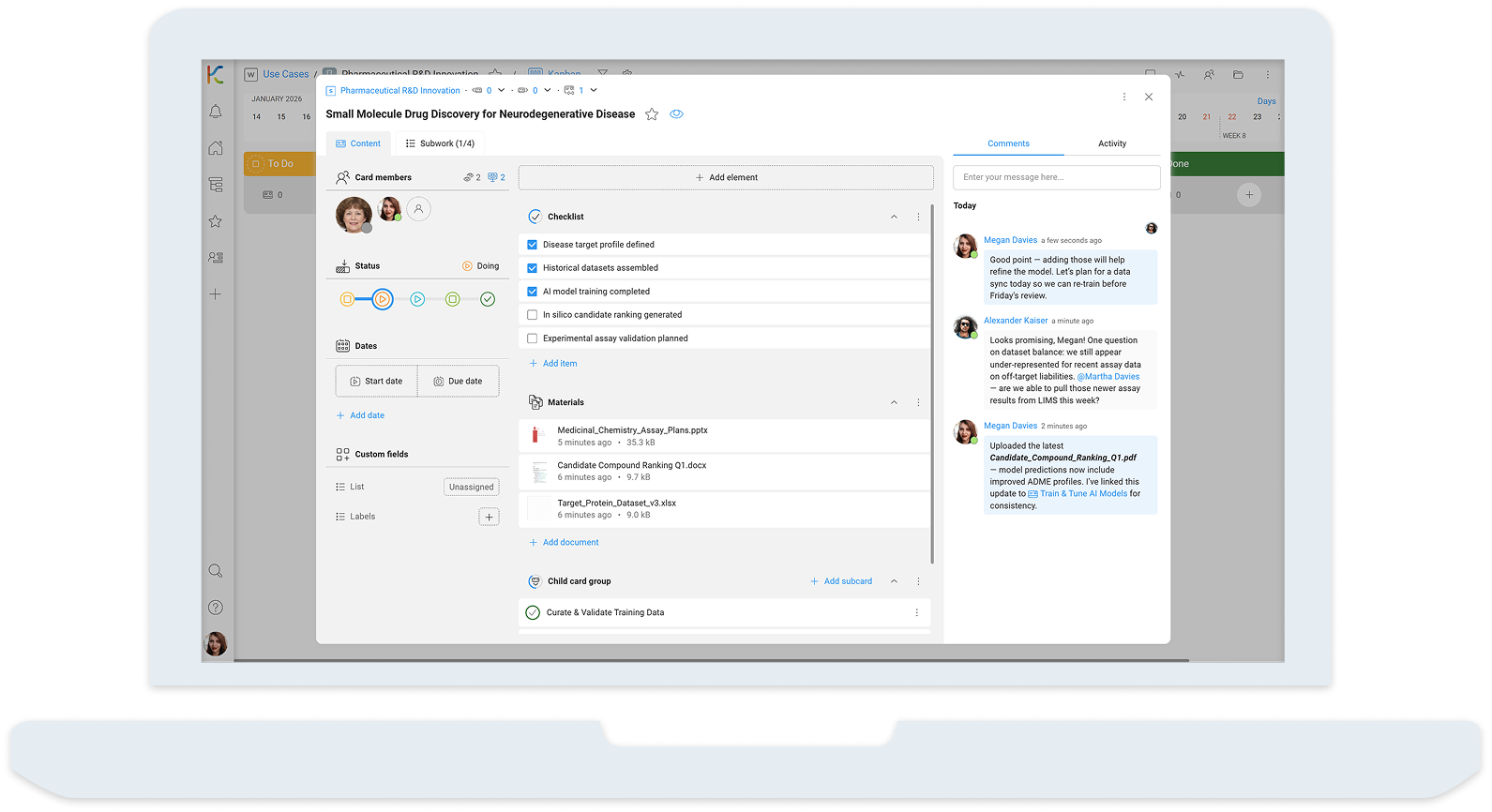
How the Organization Uses KanBo
KanBo acts as a digital innovation and compliance hub, helping the pharmaceutical organization unify global teams, structure workflows, and adapt quickly to dynamic regulatory and market conditions. Key functional areas include:
I. AI-Driven Drug Discovery Management
- Research Boards: Track milestones, hypotheses, and experiments.
- AI Progress Dashboards: Visualize machine learning workflows and discoveries.
- Task Automation: Streamline repetitive tasks and manage dependencies.
II. Regulatory Compliance & Risk Alignment
- Compliance Spaces: Centralize FDA/EMA documentation and SOPs.
- Approval Workflows: Ensure timely reviews and submission tracking.
- Notification Systems: Alert teams to regulatory updates and deadlines.
III. Clinical Trial Coordination
- Trial Phase Boards: Manage preclinical through Phase IV.
- Enrollment Tracking: Monitor patient recruitment and retention.
- Analytics Integration: Real-time trial data, adverse event tracking, and KPIs.
IV. Market Expansion & Global Strategy
- Market Entry Playbooks: Assess timelines, partners, and market access risks.
- Expansion Cards: Track country-specific requirements and legal status.
- Strategic Maps: Visualize go-to-market activities and expansion milestones.
V. Direct-to-Consumer (DTC) Engagement
- Patient Journey Boards: Coordinate engagement, education, and support.
- Telehealth Scheduling Tools: Manage appointments and follow-ups.
- Delivery Coordination: Track last-mile medication logistics and feedback loops.
VI. Resource Allocation & R&D Optimization
- Project Workload Views: Identify resource bottlenecks across departments.
- Budget Tracking Cards: Align costs with ROI-focused initiatives.
- Strategic Dashboards: Optimize time and talent allocation across pipelines.
Roadmap for Implementation
Planning and Setup
Pilot Phase
Pilot Evaluation
Full Rollout
Project Highlights
Implementation Duration:
18 months from planning to global rollout
System Integrations:
Microsoft SharePoint, Office 365, HIPAA-compliant cloud, AI analytics platforms
Performance Improvements:
30% faster AI implementation, 20% cost savings in R&D, faster compliance turnaround
Real-World Applications in KanBo
AI-Driven Drug Discovery Board
Milestone-based tracking of algorithms, targets, and preclinical outcomes.
Regulatory Compliance Workspace
Real-time documentation, approval workflows, and audit readiness.
Clinical Trial Execution Hub
Trial tracking from protocol design to patient follow-up.
Benefits of Using KanBo in Pharmaceutical Innovation
Accelerated Drug Development
Structured innovation workflows that fast-track discovery to delivery.
Real-Time Compliance Tracking
Minimize delays and errors with centralized regulatory tools.
Optimized Resource Utilization
Maximize R&D impact with transparent workload distribution.
Improved Cross-Functional Collaboration
Connect R&D, regulatory, and commercial teams globally.
Security and Installation Options
On-Premises & Government Cloud Deployment
Ensures compliance with national security regulations.
Role-Based Access Controls
Restrict sensitive investment data to authorized personnel.
Data Encryption & Audit Logging
Protects against cyber threats and ensures transparency.
KanBo – Work Coordination Platform
KanBo is a work coordination software designed to help self-organizing teams work smarter and faster. You can see KanBo in action by accessing our demonstration environment.
